Viatris 2023 Sustainability Report: Building Access Through a Global and Flexible Supply Chain
No country can make every medicine people need, and no medicine is made in every country. If a country or region relies on supply only from within their region and they experience a crisis, there is a risk people won’t get the medicines they need, when they need them. A secure supply of medicines from multiple regions and countries around the world is more reliable and at less risk of being disrupted. Global, diverse and flexible supply chains are key to timely and affordable access to medicine.
As an essential business, Viatris has taken actions to maintain a reliable supply of medicines, with special measures around critical medicines in times of volatile demand. Our ability to supply medicines and maintain high levels of service around the world is rooted in our global network of suppliers with a robust ability to manage shocks affecting any particular country or region. Our network allows us to offset risk and build resilience. In 2023, we were able to maintain a global customer service level of 90% amid volatility in demand, inflation and supply chain disruptions in general. Recently, we have experienced surges in demand, in part due to other pharmaceutical companies being out of stock, so we are increasing the frequency that we refresh our safety stock settings so that we can be more flexible to meet unmet needs.
Viatris has Supply Chain colleagues in more than 55 countries around the world, responsible for monitoring demand and supply daily. They look out over a 24-month horizon to preempt and circumvent supply gaps, collaborating with markets and manufacturing plants on cross-functional action plans.
Learn more about our global and local supply network here.
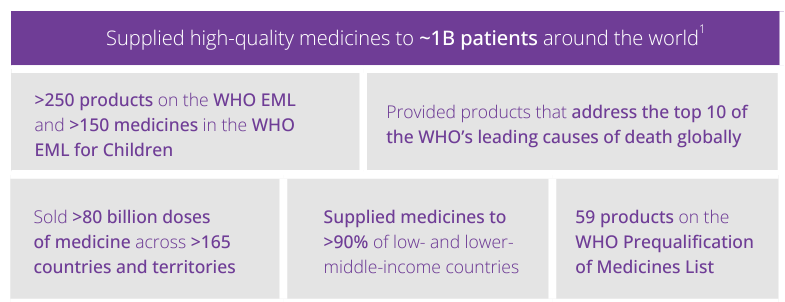
In 2023, our global customer service level was 90%.
Our customer service level metric is on-time in-full (OTIF) delivery to our customers. On-time is customer specific and measured against customer agreements. In-full is 100% of volume ordered. It is important to Viatris to measure service from our customers’ perspective.
We have invested in facilities around the world, including in lower- and middle-income countries (LMICs), to bolster our global supply chain network and support local capacity building and economic development in communities. These local facilities make up our strong global network, which is our best tool for maximizing product availability to countries across all income bands, as evidenced by our track record for reliable supply.
In Vietnam, for example, we’ve worked in collaboration with the government to enhance local production capacity as part of supporting the global supply of medicines and also supporting economic resilience in the country. Our project, one of the first innovative drug production technology transfer projects in Vietnam, started in 2015, and was approved by the Ministry of Science and Technology in 2019 for Amlor tablet (Norvasc); Lipitor 10mg, 20mg, 40mg; and Celebrex 200mg.
In India, Viatris manufactures infectious disease medicines used in programs across LMICs. To supplement this network, Viatris has invested in building a packaging and distribution facility in Zambia, which achieved WHO Prequalification of Medicines (WHO PQ) accreditation in March 2023. Finished medicines are shipped from our facilities in India to the Zambia facility for final packaging and release. The value-add of packaging in Zambia is not necessarily to improve availability in its absolute vicinity, as our existing global network continues to provide high levels of product delivery, but to help develop local capacity and experience with quality-assured production.
Goal: Provide ARV therapy equivalent to a total of 30 million patients, including more than 2 million children living with HIV/AIDS, between 2022 and the end of 2025.*
Our Progress: In 2023, we provided treatments for approximately 8.6 million patients, including more than 670,000 children living with HIV/AIDS. Since 2022, we have provided treatments for nearly 17 million adults and children.
View the full 2023 Sustainability Report.
1 The number of patients served is an estimate calculated using internal sales data (global volume of doses sold in 2023 in all markets as aligned with IQVIA standard units), divided by estimated per patient usage, which is based on treatment dose, treatment duration, and treatment adherence as estimated by Viatris Medical Affairs based on approved label indication and instructions for use, current international guideline recommendations, and common usage in clinical practice. Patients using multiple Viatris medicines may be counted as multiple patients. Certain adjustments were applied in consideration of pending and completed divestitures and to account for acceptable alternatives to the patient usage factors noted above, and rounded to the nearest hundred million. Estimates may be subject to reassessment.
*Our ability to make progress on our goals depends on several factors, some of which are outside of our control.

