Ferring Pharmaceuticals and Merck Announce Completion of Largest Clinical Trial Ever Conducted in Postpartum Hemorrhage
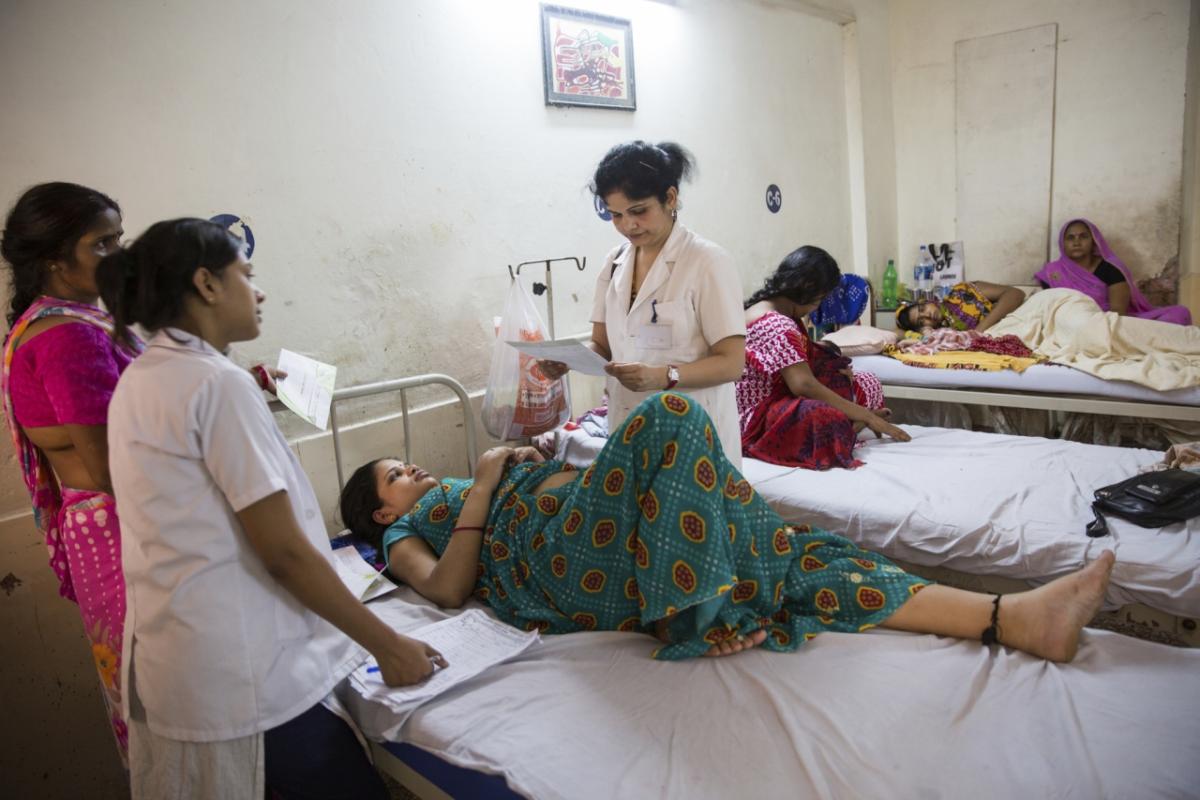
SAINT-PREX, Switzerland and KENILWORTH, N.J., February 22, 2018 – Ferring Pharmaceuticals and Merck, known as MSD outside the United States and Canada, through its Merck for Mothers initiative, today announced the completion of CHAMPION (Carbetocin Haemorrhage Prevention), a global clinical trial conducted by the Human Reproduction Program (HRP) at the World Health Organization (WHO). The CHAMPION trial is studying whether Ferring’s investigational proprietary heat-stable formulation of carbetocin could offer a new solution to prevent excessive bleeding after childbirth (postpartum hemorrhage or PPH). Involving nearly 30,000 women in 10 countries, it is the largest clinical trial ever conducted in PPH. These 10 countries include Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda, and the United Kingdom.
Each year, 14 million mothers are affected by PPH. As the leading direct cause of maternal mortality, 480,000 mothers died from PPH between 2003-2009. Even when women survive, PPH can result in the need for serious medical interventions, including surgical removal of the uterus (hysterectomy) as well as blood transfusions to address severe anemia.
The CHAMPION trial compares the effectiveness and safety of Ferring’s heat-stable carbetocin versus the current standard of care, oxytocin, for preventing PPH after vaginal birth. Oxytocin requires refrigeration during shipping and storage to prevent degradation in temperatures above 8 C. Heat-stable carbetocin may remain active long-term in hot and humid climates, and in many low- and lower-middle-income countries, cold storage is difficult to achieve and maintain.
“Despite progress towards the UN Sustainable Development Goal of reducing maternal mortality, every single day women across the world are dying unnecessarily from childbirth complications such as PPH. Timely administration of effective medicines can avoid the maternal deaths that occur due to excessive bleeding after childbirth,” said Mariana Widmer, Technical Officer, Maternal and Perinatal Health, WHO. “If the results of the trial for heat-stable carbetocin are favorable, this collaboration between private life sciences and the global public health community could help save women’s lives worldwide.”
“Using our established expertise in Reproductive Medicine and Women’s Health, we strive to find innovative treatments that will help to dramatically reduce the number of mothers dying as a result of childbirth,” said Professor Klaus Dugi, Chief Medical Officer, Ferring Pharmaceuticals. “We are looking forward to seeing the results from the CHAMPION trial and hope that the learnings will usher in a new era in the prevention of PPH.”
If the results of the CHAMPION trial are favorable, Ferring plans to seek registration of heat-stable carbetocin on a broad basis. If approved, Ferring would manufacture the product and it would be provided to the public sector of low- and lower-middle-income countries at an affordable and sustainable access price. Results from the trial are expected to be presented and published during the second half of 2018.
“The CHAMPION trial has the potential to change the paradigm in how we save more mothers from dying during childbirth,” said Julie L. Gerberding, M.D., M.P.H., executive vice president & chief patient officer, Strategic Communications, Global Public Policy and Population Health at Merck. “Through Merck for Mothers, we provided our company’s scientific expertise and financial resources and ultimately, we hope to make a sustainable impact on the health of mothers, families and communities.”
About the CHAMPION trial
CHAMPION (Carbetocin Haemorrhage Prevention), the world’s largest clinical trial in postpartum hemorrhage, is being undertaken to compare the effectiveness and safety of heat-stable carbetocin to oxytocin in the prevention of postpartum hemorrhage after vaginal births. The trial conducted by the Human Reproduction Program (HRP) at the World Health Organization enrolled nearly 30,000 women in 10 countries including Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda and the United Kingdom. Heat-stable carbetocin was researched and developed by Ferring Pharmaceuticals and the CHAMPION trial was funded by Merck for Mothers.
About Ferring Pharmaceuticals:
Ferring Pharmaceuticals is a research-driven, specialty biopharmaceutical group committed to helping people around the world build families and live better lives. Headquartered in Saint-Prex, Switzerland, Ferring is a leader in reproductive medicine and women’s health, and in specialty areas within gastroenterology and urology. Ferring has been developing treatments for mothers and babies for over 50 years. Today, over one third of the company’s research and development investment goes towards finding innovative and personalised healthcare solutions to help mothers and babies, from conception to birth. Founded in 1950, Ferring now employs approximately 6,500 people worldwide, has its own operating subsidiaries in nearly 60 countries and markets its products in 110 countries. Learn more at www.ferring.com and @Ferring, or connect with us on Facebook, Instagram and LinkedIn.
About Merck for Mothers
Every day, approximately 830 women die from preventable causes related to pregnancy and childbirth. Merck for Mothers is a 10-year, $500 million initiative to create a world where no woman dies from complications of pregnancy and childbirth. Drawing on the company’s history of discovering innovative, life-saving medicines and vaccines, Merck for Mothers is applying the company’s scientific and business expertise – as well as its financial and human resources – to reduce maternal mortality around the world. Learn more at http://www.merckformothers.com/ and @MerckforMothers.
About Merck
For more than a century, Merck, a leading global biopharmaceutical company known as MSD outside of the United States and Canada, has been inventing for life, bringing forward medicines and vaccines for many of the world’s most challenging diseases. Through our prescription medicines, vaccines, biologic therapies and animal health products, we work with customers and operate in more than 140 countries to deliver innovative health solutions. We also demonstrate our commitment to increasing access to health care through far-reaching policies, programs and partnerships. Today, Merck continues to be at the forefront of research to advance the prevention and treatment of diseases that threaten people and communities around the world - including cancer, cardio-metabolic diseases, emerging animal diseases, Alzheimer’s disease and infectious diseases including HIV and Ebola. For more information, visit www.merck.com and connect with us on Twitter, Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Kenilworth, N.J., USA
This news release of Merck & Co., Inc., Kenilworth, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline products that the products will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s 2016 Annual Report on Form 10-K and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).
Merck Contact:
Claire Gillespie
(267) 305-0932
Ferring Contacts:
Bhavin Vaid
(41) 58 301 0952
Lindsey Rodger
(41) 58 451 4023

