4 Strategies to Help Your Facility Respond to the Changing Animal Health Market
by Kofi Dalrymple, Ph.D., Senior Process Engineer
Originally published by CRB Group
In many ways, there has never been a better time to develop and manufacture therapies for the global animal health market. Analysts expect this $38.5 billion industry to grow by up to 5.7% annually between now and 2023.
Recent high-profile mergers and acquisitions account for some of this growth, but much of it is the result of intangible shifts in cultural attitudes. Today’s pet owners expect the same quality and reliability from animal health products that they do from human pharmaceuticals. Agricultural companies, meanwhile, are responding to consumer pressures by demanding more innovation and improved health outcomes from the therapies they invest in for their livestock. In the midst of this dynamic consumer environment, enterprising manufacturers have the opportunity to thrive.
As with most opportunities, though, this one comes with its share of challenges. Many legacy companies are grappling with aging infrastructure and limited capacity. Startups face a related challenge—they need to know how best to protect their investment with a facility designed for growth and continuous change. For both types of companies, and everyone in between, the solution lies at the intersection of four emerging business strategies:
- Improved flexibility through multi-modal technology
- Faster speed-to-market through data-driven process optimization
- Greater ROI through innovative drug formulations
- Broader market-share through globally-driven regulatory strategies
Every company will have its own formula for success, integrating these approaches with their existing processes and practices in a way that makes sense in their context. In this article, we’ll help you find your best path forward by taking you inside each strategy, examining its strengths, its challenges, and the ways in which it could help you claim a greater share of the “new” animal health marketplace.
1. Improved flexibility through multi-modal technology.
In my role leading process design for upstream and downstream technology with many of CRB’s animal health clients, I often hear the same questions:
- How can we diversify our product portfolio without expanding our facility?
- How can we increase the value of each square foot of our existing operations?
- How can we ready ourselves to respond more quickly to a promising new product or a shift in the marketplace?
- How can we transition more smoothly between products without risking non-compliance?
These questions boil down to one important fact: improving the diversity and flexibility of your product pipeline is critical to business success, now more than ever.
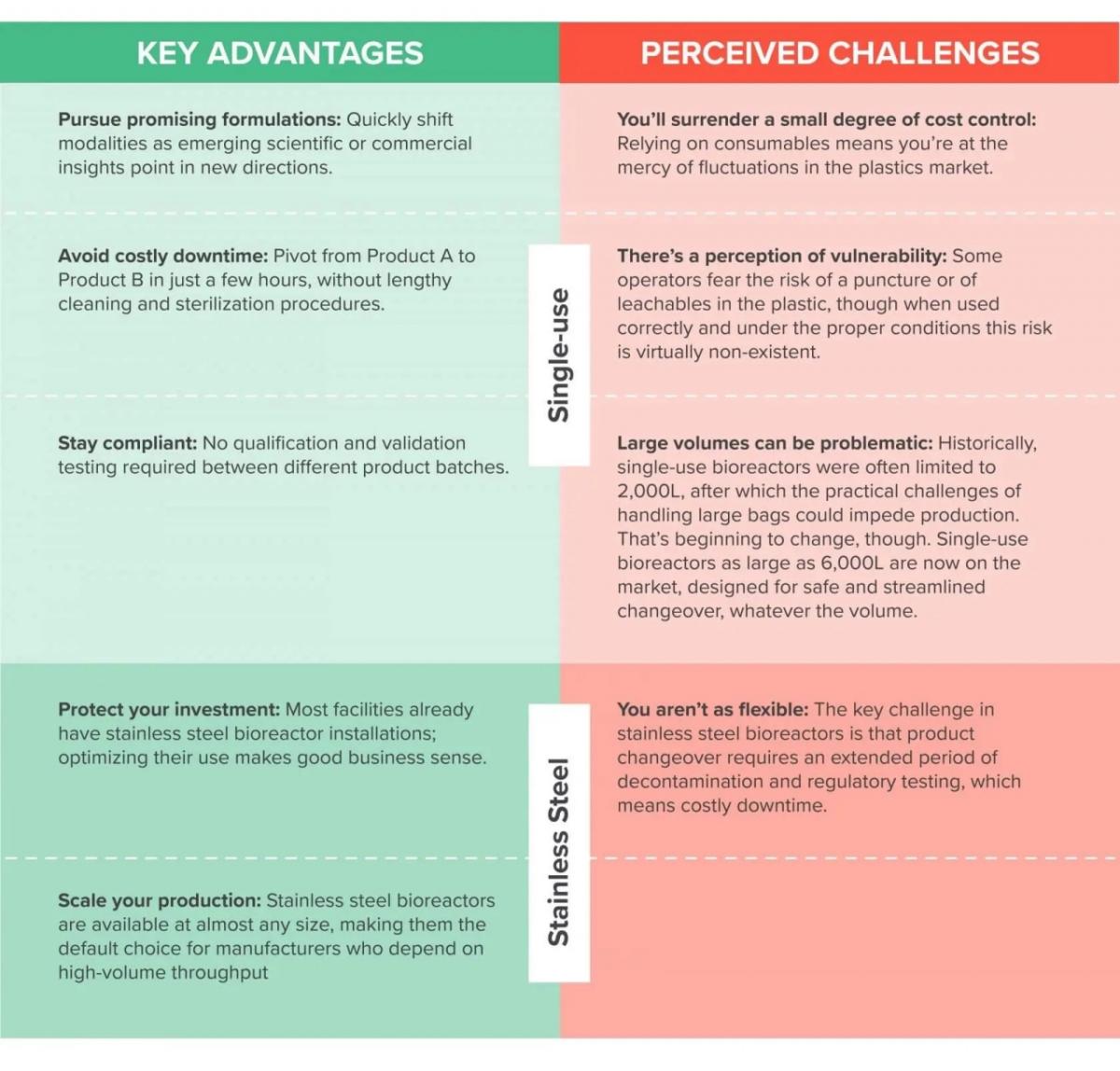
This has led many forward-thinking animal health companies to embrace systems that can pivot from one product modality to another with minimal downtime. Single-use technology is one of the most popular examples. It offers a compelling alternative to the inefficiency of sterilizing stainless steel equipment in place between products, and it eliminates the risk of exposing cleanrooms to contamination by introducing recycled consumables. The upshot is improved agility in the production space, making it much easier—and far faster—for companies to respond to internal and external pressures.
There’s no magic bullet, though. Like every technology in your facility, the value of single-use systems comes from how well you’re able to balance its many advantages with its risks or challenges, such as the cost of single-use consumables.
This isn’t about choosing one technology over another. The best facilities that I’ve seen are those with a bespoke hybrid approach, in which stainless steel and single-use technologies are put to work in an integrated process flow that leverages the best of each while minimizing their risks and challenges.
For example, one cell culture may begin its growth in a single-use bioreactor before transferring to a stainless-steel bioreactor to complete its processing cycle . This can be a complicated dance, drawing on the principles of quality by design (QbD) and the unique nature of your product pipeline, but getting it right can have an enormous impact on how easily and reliably you navigate this turbulent marketplace.
2. Faster speed-to-market through data-driven process optimization.
Some buzzwords are all air, but “digital transformation”—perhaps the buzziest of them all—has very real substance and will help the animal health industry achieve incredible outcomes in the near future. Already, veterinarians are using big data to improve epidemiology, farmers are benefitting from data-driven breeding programs, and pet owners are tracking their furry friend’s health through all kinds of wearable tech.
Over in the animal drug development and manufacturing industry, these advances are just beginning to find their footing. A few years from now, they’ll be a mainstay; data-driven instrumentation and control mechanisms will be part of every developmental pipeline and crucial to every production process. To get a head start and ensure that your own internal workings are calibrated for the imminent data transformation, here’s what to do.
Understand how your process works.
Like finding the ideal balance between stainless steel and single-use technology, this is about applying a QbD approach to your process architecture—one that reflects your company’s internal drivers.
Data analytics will help you understand your process right down to its atomic parts. Once you do that, you can test and analyze the multivariate factors that impact that process. You have the knowledge you need to design experiments, make improvements, and introduce new technologies, while fully aware of which factors are impacting your product’s quality, and in what way. From this seat of control, you can begin to make a truly meaningful change from the inside out, driving your operations to new efficiencies.
Turn that understanding into action.
Based on what you uncover in that analysis of your process, you can enlist scientific and engineering principles—guided by data-driven insights—to improve your productivity.
Take the mixing dynamics inside a bioreactor, for example. Historically, process development teams relied largely on empirical testing to understand how a substance would react to a given environment. Today, computer simulations offer predictive capabilities by allowing teams to model and resolve critical process parameters including, for example, cellular behavior inside a bioreactor. How will a substance respond to the force dynamics of an impeller? What are the distribution dynamics of an acid or nutrient feed introduced from an opening at the top of a bioreactor?
Data generated through these simulations become valuable insights as teams strive to improve the process overall, or develop an efficient, high-yield process for a new product. Simulation can be a real cost-saver, allowing you to explore a wide range of possibilities and variables in a way that’s simply not practical with traditional experimentation.
The same is true in terms of understanding how to improve yield. New instrumentation allows manufacturing teams to measure how much product is generated throughout the fermentation lifecycle; this is an improvement on historic approaches to calculating yield, which may rely on imperfect measurements of cell concentration only. With online monitoring, you know exactly how much product you’re making at any given time, which means you can get more from every batch.
These are just two examples of data “at work” in the manufacturing lifecycle. As technology improves and data analysis becomes more entrenched in everyday decision-making, more examples will emerge, giving innovative and enterprising animal health manufacturers the opportunity to make very interesting things happen—and fast.
3. Greater ROI through innovative drug formulations.
Asthma inhalers for horses. Cancer therapies that jump the divide between dogs and people. These are just two examples of the many synergies linking human and animal pharmaceuticals, and more are emerging every day. Finding opportunities to leverage those synergies in the accelerated pursuit of better, more effective animal therapies is a popular objective, and a lucrative one—provided developers and manufacturers are prepared for the challenges that may await them in this pursuit. The nature of those challenges has much to do with the target market.
- In the companion animal market: A decade ago, pet owners largely accepted that Fido ’s annual vaccine might precipitate a temporary intramuscular lump (rash or bump), the result of a mild allergic reaction to impurities. Today, the culture around pets and medications has changed; owners are willing to pay more for a “safer” product, which means a product made with the same level of care as a human pharmaceutical drug. This challenges animal health companies to modernize their downstream drug manufacturing processes to offer a more purified product. For many legacy companies whose aging facilities were never designed for such elevated expectations, this is a problem.
- In the agricultural market: Innovative drug delivery systems are helping livestock owners design superior drug treatment programs. Dissolving a powder in the water system or distributing aerosolized particles is a faster and more humane way to vaccinate a herd, for example, and it uses drug products with a longer shelf life—another advantage for the agricultural operator. The risk, though, is that developing and manufacturing these pioneering products can require significant investment, which means charging the end consumer a premium. Agricultural operators, already jostling for market share in an intensely competitive field, are reluctant to hurt their competitive advantage by raising their prices and therefore may not pursue the newest innovations, no matter how effective they may be. For manufacturers, this raises an important question: how can they offer “innovative, next-generation products” without charging “innovative, next-generation” prices?
The answer to both of these challenges comes down to a drug company’s success at improving and streamlining their internal processes. Thereby uncovering hidden cost efficiencies—a huge advantage to the drug manufacturer and to the end consumer, both from a pricing perspective and in terms of pipeline quality and reliability. By improving your internal processes through some of the principles discussed in this article—multi-modal manufacturing, data-driven process optimization—you’ll have the infrastructure you need to develop differentiated drug formulations and the efficiency you need to market them competitively.
4. Broader market-share through a global regulatory strategy.
The opportunities I’ve discussed won’t do you much good unless you’re able to secure and maintain compliance. There’s where having a robust regulatory strategy comes into focus.
A regulatory strategy is especially key as more US-based companies vie for global distribution, which requires a good understanding of the nuances of multiple regulatory environments. Are you aiming for market-share in the EU? Russia? Australia? The answer will help you future-proof your investment in any new project, whether an upgrade to your existing manufacturing suites or a whole new greenfield facility. The early and continuous involvement of a regulatory sciences and submissions team, whether your own or through a partnership, is essential.
The animal health industry is undergoing a significant shift toward new and versatile technology.
Navigating that shift takes experience and a deep understanding of the technologies in play.
We’re here to help. I’m just one part of an extended animal health team at CRB—a team whose experience has been supporting this industry for over 25 years. Our partners include Merck Animal Health, Boehringer Ingelheim, and Elanco, among several others. Let us help your animal health company, large or small, leverage the latest knowledge and the best technology to accelerate your success in this exciting and high-growth industry.